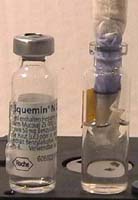
 .
. .
. .
.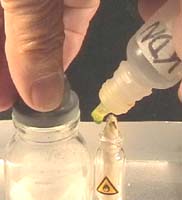 .
.
 .
.Experiment
1: Make a micro spirit burner from a Liquemin
bottle, re-label it
1.
Halve a piece of tissue paper, draw a line 2 cm above one side with a black
felt pen.
2.
Roll the paper to make a wick,
3. Left photo.
Dip the wick into the bottle filled with methylated spirit. Let it rise.
Observation?
More
about this.
Experiment
2: Light you micro burner, stop the air supply
1.
Add some water to your tray, light your burner, place it into the tray.
Photos
2 and 3: 2. Stop the air supply by covering the flame with a
dry infusion bottle.
Observation?
Explanation:
Burning
is a chemical reaction:
Methylated
spirit and air are consumed, a a new substance is formed: water.
Experiment
3: Repeat experiment 2. Test for carbon dioxide in the
consumed air
1.
Cover the flame of the burner again.
2.
As soon as the the flame fades, lift the bottle and stopper it immediately.
Photo
4: Add 10 drops of lime water to the consumed air, close again, shake
the bottle.
Observations:
right photo
Explanation:
The
chemical reaction of methylated spirit and air resulted in a second substance:
carbon dioxide..