 ....
.... ...
...
Photo 1
 Introduction
Introduction
1 Pfennig coins - once
the smallest unit of German money- were made of steel coated by copper.
These coins are useful not only as 2.0-gram weights on a scale [6] but
also as anodes, cathodes and as
source of copper salt solution. This solution will be
obtained by one of
the most dramatic chemical changes present in any curriculum of
the world:
"While reading
a textbook of chemistry, I came upon the statement `nitric acid acts upon
copper` and I determined to see what this meant.Having located some nitric
acid I had only to learn what `act upon` meant. In the interest of
knowledge I was even willing to sacrifice one of the few copper cents then
in my possession. I put one of them on the table, opened a bottle labeled
`nitric acid`, poured some of the liqid on the copper coin, and prepared
to make the observation. But what was this wonderful thing which
I beheld? The cent was already changed, and it was no small change
either. A greenish-blue liquid foamed and fumed over the cent and over
the table. The air became colored dark red. How could I stop this? I tried
by picking the cent up and throwing it out of the window. I learned another
fact: nitric acid acts upon fingers. The pain led to another unpremeditated
experiment. I drew my fingers across my trousers and discovered: nitric
acid acts upon my trousers...." (and also upon fingers giving them a
yellow color) [7].
By doing
this coin experiment on microscale all above abservations can be reproduced
by using only 1 microdrop (50 microlitres) of nitric acid. All other
recommended safety precautions (goggles, waste manipulation, ventilation
etc) should be strictly aoolied. The corroding and dyeing effect of nitric
acid may be demonstarted with an egg instead of the skin.
In many countries Cola
cans are made from two different metals: Only the closure consists of aluminium
while the rest of the can is steel („FE“) double coated with plastic.
The complete can
after rinsing
is used for the collapsing can experiment [9]. The can is also used
to demonstrate the burning and explosion of hydrogen [10], [11]: The upper
side of the can is cut off, and the can is restored into its previous
shape again and its bottom is perforated by a tiny hole. For further
Microscale Chemistry Experimentatio the can is cut into small pieces.
In the following experiments
Cu, Fe and Al
will be used to explore basic concepts of electrochemistry.
Beads of different colors
and sizes will be used as models for atoms, ions and electrons [12].
1. Direct electron transfer from copper atoms in a coin to the nitrate ions
 ....
.... ...
...
Middle: The direct
transfer of two electrons from a copper atom to two nitrate ions is visualized
by beads of different sizes and colors. The orange bead with the two micro
beads is a model of a copper atom. The two blue beads each of them welded
with three red beads show models of two nitrate ions.
Right: The models
illustrate the electrons (white micro beads)
transfered
from the copper atom to the nitrate ions.These two electron acceptors
will immediately continue reactions resulting in the nitrogen dioxide observed.
Reduction:
2 [NO3] -(aq) + 2e- +4 H
+ (aq) --> 2 NO
2
(g) + 2 H2O(l)
Oxidation:
Cu(s) ---> Cu2+(aq) +2 e-
Redox reaction:
Cu(s) + 2 [NO3]-(aq) + 4 H+(aq)
--> Cu2+(aq) +
2 NO
2
(g) + 2 H2O(l)
Cu(s)
+ 4 HNO 3 --> Cu(NO
3)
2 + 2 NO2
+ 2 H2O(l)
2. Direct electron transfer from aluminium atoms of the can closure to the copper ions (microscope)
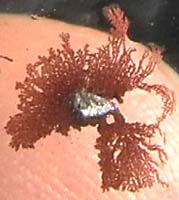 Photo 2:
Photo 2:A drop of the copper nitrate
/ table salt solution obtained from the Pfennig coin is placed on a
microscopic slide. A tiny piece of aluminium - cut from the closure of
a Cola can- is pushed into that drop.
It can be observed that
small trees in the colour of copper are branching out.
3. Electron transfer from atoms of activated aluminium to water molecules
Photo 3 Aluminium of a can closure activated by a Pfennig coin reacted with water
A can closure is connected
to a Pfennig coin by a crocodile clamp and placed in a blister with sea
water solution.
After some hours a white gelatinous precipitate (wp) appears below
the can closure. Gas bubbles (b) can also be seen at the rim of the
coin.
The observations are
the result of an indirect electron transfer from aluminium to water
through copper.
Aluminium is the electron donor , water being reduced to hydrogen:
Reduction:
6 H2O(l) + 6 e- -->
6 OH-(aq) + 3 H2(g
4. Forced electron transfer from Cl - to Cu2+ ions during electrolysis
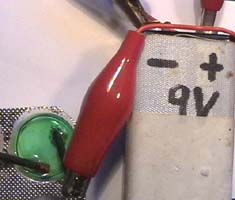 .
. .
.
Photos 4.1 - 4.3
Electrolysis of
a mixture of copper salt solution and table salt (1 + 2), testing for
chlorine (3)
The solution from experiment
1 is rinsed into a blister by adding drops of concentrated table salt solution.
Left: Two pencil leads –connected with a 9-Volt battery- dip into
this solution. Middle: A layer with the color of copper
appears on the negative (left) electrode, gas bubbles are around the positive
electrode.
Cathodic reduction:
Cu2+(aq) + 2e- -->
Cu(s)
Anodic oxidation:
2 Cl-(aq) --> Cl2 (g)
+ 2e-
Redox reaction: Cu2+(aq) + 2
Cl -(aq) --electrolysis--> Cu(s)
+ Cl2 (g)
Right: This photo
shows the result of testing for chlorine: A white tissue paper wetted
with colorless potassium iodide solution was dipped into the right side
of the blister. This reagent for chlorinet turns brown.
A second redox reaction takes place during the test for chlorine:
Cl 2
+ 2
l- ----->
2 Cl
-
+
l2
5. Spontaneous electron transfer from Cu to Cl2 on carbon electrodes in a Galvanic Cell
6. Galvanic Cells with a Pfennig (Cu) and the two different metals (Al and Fe) from a Cola can
Photo 6
Galvanic Cell potential
between Cola iron and copper of a Pfennig
7. Batteries of Galvanic Cells made from can metals in vials with salt bridges
Photo 7
Potential between a battery
of three Galvanic Cells made of two
Cola can metals (Al, Fe)
8. Electrolysis of table salt solutions with anodes made of two Cola can metals
 ..
.. ..
..
Photos 8.1 - 8.3
Electrolysis of salt water
with aluminium anode (1) and with iron anode (2 and 3
)
Literature
[1]
E.C. Grey, Practical Chemistry by Micro-Methods, W. Heffer &
Sons, Ltd., Cambridge 1925
[2] M.K.
El-Marsafy et al, The microscale chemistry laboratory technology its
implications on the future education, Book of abstracts symposium on science…,
S. 18 – 20, American University Cairo 1995
[3] M.K.
El-Marsafy, P. Schwarz, Microscale chemistry experimentation, 119
Seiten englisch und arabisch, Kairo 1996
[4] P.
Schwarz, Chemische Unterrichtsexperimente im Internet, CHEMKON/8. 1,
(2001) 47
[5] P. Schwarz,
Nachhaltiges Erziehen durch mikrochemisches Experimentieren im Kindergarten,
Chemie und Schule 2 (2001), S. 13f
[6] P. Schwarz,
Beobachten, Messen, Experimentieren im Kindergarten der Sharm El Sheikh
Language School, CHEMKON/8. 3, (2001) 169f
[7] Brown LeMay Bursten, Chemistry the central science, Prentice
Hall International, Inc., New Jersey 2000
referring to Ira Remsen, Chemistry Textbook 1901)
[8] http://www.micrecol.de/water4.html
[9]
http://www.micrecol.de/water4a.html
[10] http://www.micrecol.de/afuel11.html
[11] V. Obendrauf,
Döschen hüpf! Die pfeifende Dose im Schülerversuch, Chem.
Sch. 15 (2000) Nr. 4, S. 8 - 12
[12] http://www.micrecol.de/elecE5c.html
About the authors
M.K. El-Marsafy http://www.micrecol.de/ marsafyE.html P. Schwarz http://www.micrecol.de/ peterE.html