 ...........
........... ...........
...........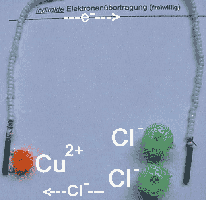
5. Spontaneous electron transfer from Cu to Cl2 in a Galvanic Cell
After finishing the electrolysis
of the copper salt / table salt solution in experiment 4 the battery
is removed and the electrodes are connected to a digital multimeter. It
reads a pontial of 1.10 V (1 )and a current of 1969 µA (2).
Bead models (3) illustrate the electrochemical and electrical
steps of this spontaneous indirect electron transfer from Cu to Cl2.
Reduction: Cl2(g)
+ 2e- --> 2 Cl-(aq)
Oxidation:
Cu(s) --> Cu2+(aq)
+ 2e-
Redox reaction: Cu(s) + Cl2
(g) --> Cu2+(aq) + 2 Cl-(aq)