Microscale Chemistry Experimentation for Teachers
30.
More
modelling for explanation of NaCl synthesis

Energy is consumed to break the metallic
bonds of solid sodium Na(s).
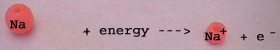
Energy is also needed for the ionisation
of the sodium metals (Na).
The resulting positive sodium ions
(Na+) have a smaller ionic radius than the Na atoms:
The nucleus has to attract one electron less.

Bonding energy is to be supplied to transform
chlorine molecules (Cl2) into chlorine atoms (Cl)

Each electron which was donated by one
Na atom during its ionisation is accepted by one chlorine atom (Cl).
During this step Cl atoms are transferred
into negative chlorine ions (Cl-).
While the radius of Na was decreasing
during ionisation, the ionic radius of Cl is increasing during ionsisation.
The Cl- ions have a bigger
ionic radius than Na+ ions.
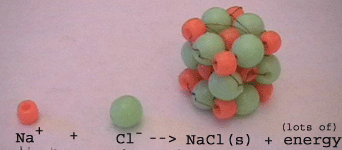
Formation of a NaCl crystal lattice
(final step)