 ...
... ...
...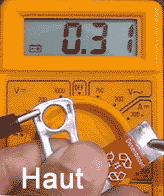
Me09 Electricity by redox reaction of the two Cola can metals
 ...
... ...
...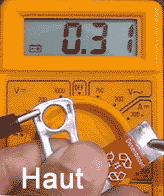
Me09 Electricity by redox reaction of the two Cola can metals
In the previous
experiment with metals of a Cola can aluminium was a better
electron
donor
than iron: Its reaction with hydrochloric acid was stronger.
In the following experiment
you will observe that aluminium can also indirectly transfer electrons
in an electric circuit.
What
you need: Cola
can (recycling icon FE), scissors, sand paper, 2
insulated copper wires with 4 crocodile clips, digital multimeter,
fruit (apple), salt water.
Experiment:
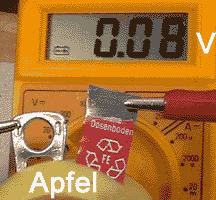
Cut a piece of steel 15 x 60 mm (Fe) from a Cola can, sandpaper
its upper and lower ends, connect it to the crocodile clip of the red wire.
Fix the closure of the Cola can to the white wire.
Clip the white wire to the lower
terminal of the multimeter, the red one to the terminal in the middle.
* Use the free ends of the two
metals to puncture an apple (left), to dip it into salt water (middle)
and to press it between thumb and fore finger (right).
Observations:
The multimeter reads voltages
between 0.1 und 0.3 Volt. A current flows between the two metals.
Explanations:
These
three divices are
Galvanic Cells. The details
of the chemical reactions you will learn by the following
experiment.