 ........
........ ........
........
 ...
...
Orbital:
" ... usually represented by a picture that shows the boundary of the space
surrounding the nucleus of an atom within which there is some finite probability
(such as 90%) of finding the electron" or two paired electrons.
S.N.Ege, Organic
Chemistry, Structure and Reactivity, Heath and Company, Lexington, Massechusetts,
19943.
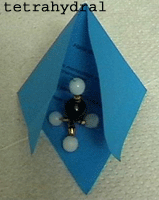
Photos
1-3: Three different hybridized atomic
orbitals (AO) of
the C atom modelled by black beads and microbeads.
Right:
Molecular
orbitals (MO) formed
by the overlap of sp3 hybrid AO with s-Orbitals of four H atoms.
A
tetrahedral structure of the metahne molecule is formed.