 .1
.1 .2.
.2. .3..
.3.. 4
4 5..
5.. .
.
salt15 Coloured
copper ions from malachite decomposition



* Photo 1: In the
previous experiment you have made a green copper
salt solution by the reaction of a piece of malachite with hydrochloric
acid.
* Photo 2: Three drops of this solution can
be used again:
* Photo 3: Dilute
with the drops in the blisters with distilled water .
* Add table salt (NaCl)
to blister 2 and a drop of ammonia solution (NH3) to blister
3.
Observation
Photo 3: Water causes a colour change from green to blue,
Photo 4: NaCl from blue to green and ammonia produces a dark blue
colour .
Photo 5: After
drying up blue crystals can be seen in blister 1, colourless cubes and a
brown solid in blister 2, a light blue solid in blister
3.
Explanation
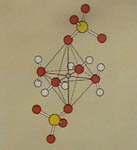
Right photo:
Copper salts contain crystal water like glassy gypsum and cobalt chloride
does. In this sketch of a model the copper ion (small grey ball) is in the
center of an octahedron (double pyramid with 8 planes).
The Cu ion is surrounded
by water molecules on four sides and by 2 sulfate (SO4) ions
on the top and below.
In aqueous solution these
sulfate ions are replaced by two more water molecules. These 6 water molecules
bonded to the Cu2+ ion in the center are called Ligands, the coloured ions are complex ions.
The colour changes to
green and to blue can be explaned by exchange of ligands:
Photo 4: Water molecules
are repalaced by a chloride ion or by 4 ammonia molecules.
Photo 5:
During evaporation of water
solid copper chloride Cu[H2O)4]Cl2 crystallizes in blister 1 and 3.
The cubes in Blister 1 are NaCl crystals, the brown colour can be explained
by exchange of water molecules by chloride ions as ligands.
back............ go on.............first published:
25.10.2001.............. ......last modification: 25.03.2007
 .1
.1 .2.
.2. .3..
.3.. 4
4 5..
5..
 .1
.1 .2.
.2. .3..
.3.. 4
4 5..
5..

