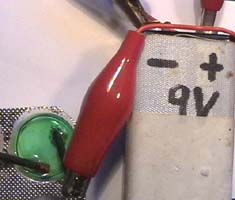 .
. .
.
Photos 4.1 - 4.3
Electrolysis of a
mixture of copper salt solution and table salt (1 + 2), testing for chlorine
(3)
4. Forced electron transfer from Cl- to Cu2+ ions during electrolysis
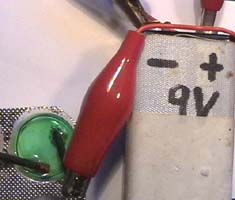 .
. .
.
Photos 4.1 - 4.3
Electrolysis of a
mixture of copper salt solution and table salt (1 + 2), testing for chlorine
(3)
The solution from experiment
1 is rinsed into a blister by adding drops of concentrated table salt solution.
1 Two pencil leads
–connected with a 9-Volt battery- dip into this solution. 2
A layer with the colour of copper appears on the negative (left)
electrode, gas bubbles are around the positive electrode. 3 4 shows
the result of testing for chlorine: A white tissue paper wetted by a colourless
potassium iodide solution turns brown because iodide was oxidised to iodine
by chlorine.
Reduction:
Cu2+(aq) + 2e- --> Cu(s)
Oxidation:
2 Cl-(aq) --> Cl2 (g) + 2e-
Redox reaction: Cu2+(aq)
+ 2 Cl-(aq) --> Cu(s) + Cl2
(g)