Microscale Chemistry Experimentation for Teachers
 ..
.. ..
..
21.
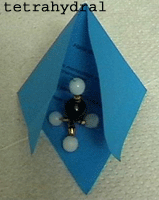
CH4 orbital model (sp3 hybridization)
Left
Four methane molecules are modelled from their atoms by connecting 1 black
plastic
bead and 4 white ones:
a and b are space filling models,
c and d are modifications of a ball-and stick model.
a was
assembled by welding the beads, b
- d by threadening the beads on copper wire.
In the second method of
assembling microbeads can be included as models of electrons or
electron pairs.
In model c
each of the bonding electrons of H is modelled by a golden microbead, the
four of C by white ones.
In model d each of
the 4 bonding pairs of electrons shared by
H atoms and the C atom is modelled by a longer microbead.
Middle: Model
of the tetrahedral arrangement in a sp3
hybridized
C atom. (These four hybrid atomic orbitals are used to bond to the H atoms
to form four equivalent C-H bonds).
Right: Methane
model
d is placed into a regular tetrahydron to visualize the tetrahydral
shape of methane molecules.