.
 .
.
Photo 3.1 - 3.3
A Pfennig coin activates
a can closure to react with water
3. Electron transfers from activated aluminium to water molecules
.
 .
.
Photo 3.1 - 3.3
A Pfennig coin activates
a can closure to react with water
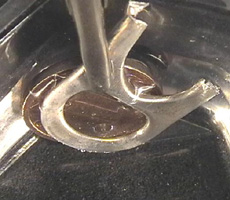
1: A can closure -
separated by a piece of transparent plastic – is placed on a Pfennig coin.
The two metals are connected by a crocodile clamp. 2: This metal
combination stands in a small honey packaging with concentrated table salt
solution. 3: After some hours a white gelatinous precipitate is
observed in front of the can closure (Al) as well gas bubbles at their
rims.
 The
two metals in the salt solution act as a Galvanic Cell.
The
two metals in the salt solution act as a Galvanic Cell.
The observations are the
result of a direct electron transfer from aluminium to water. A
contact corrosion of aluminium takes place.
[Beside this reaction an
indirect
electron transfer from aluminium via coin as cathode of the Galvanic Cell
is postulated, because gas bubbles (Photo) can also be observed at the
rim of the Pfennig coin if the table salt solution is replaced by 0.5 M
sulfuric acid.]
Reduction:
6 H2O(l) + 6 e- --> 6 OH-(aq)
+ 3 H2(g)
. . .
.